https://doi.org/10.1021/acsaom.4c00330.
Abstract
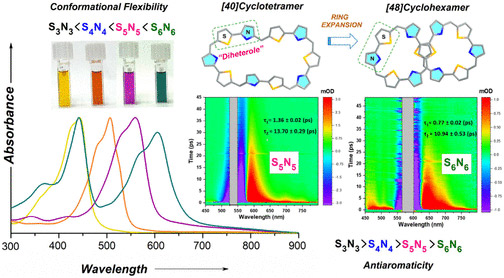
Two large-flexible porphyrinoids [40]pentathiadecaphyrin(1.0.1.0.1.0.1.0.1.0) S5N5 and [48]dodecaphyrin (1.0.1.0.1.0.1.0.1.0.1.0) S6N6 were obtained through Lewis acid catalyzed condensation of thiophene containing diheterole. The single crystal X-ray structure of S6N6 revealed a twisted “figure eight” conformation whereas the optimized structure of S5N5 displayed a coplanar arrangement of thiophene and pyrrole rings. Various spectral and theoretical studies along with the photophysical investigation of the SnNn (n = 3–6) series suggested that the higher order systems (S5N5 and S6N6) were deemed to be nonaromatic due to their nonplanar conformations. The transient absorption studies revealed a strong dependence on the electronic structure with conformational flexibility due to the expansion of the macrocyclic core. The internal conversion processes become significantly fast in higher order macrocycles SnNn (n = 5–6). These macrocycles are also shown to be promising candidates for nonlinear optical materials.